+33 (0) 3 20 16 91 40
Studies from medical biology studies

AGLAE processes data from series of proficiency tests and draws in particular information about the analytical methods or interlaboratory dispersions. These studies aim to inform laboratories. Besides, they can help understand the differences or observations laboratories may also observe.
The following studies have been conducted in medical biology:
- Practical applications of precision models
- Data dispersion in urine cytology
- Time-to-positivity in blood culture
We invite you to send us your comments on these documents.
Practical applications of the precision models in urine cytology
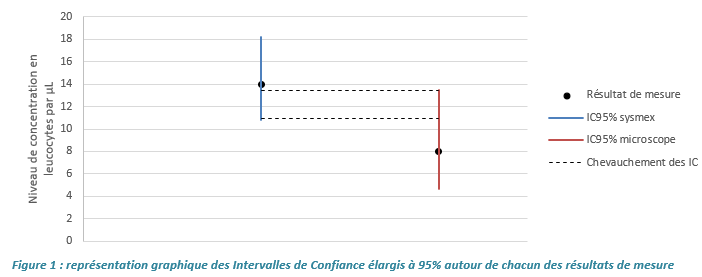 This document follows the publication presenting the expected dispersion models at different levels of white and red blood cells' concentrations. It presents practical cases and recommendations to interpret at best your data. The applications enable to answer the following questions:
This document follows the publication presenting the expected dispersion models at different levels of white and red blood cells' concentrations. It presents practical cases and recommendations to interpret at best your data. The applications enable to answer the following questions:
- How can simple acceptability criteria be obtained for use in staff qualification?
- How can repeatability and reproducibility data obtained from a method performance evaluation be verified?
- How can data obtained using two different techniques or on different technical platforms be compared?
- How can I assess my initial estimates of measurement uncertainty in cytology?
Download this part of the study on applications pratiques en cytologie urinaire (in French)
Data dispersion in urine cytology
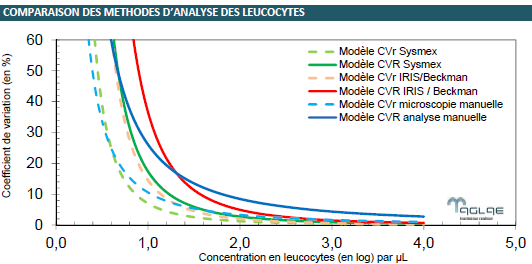
AGLAE has been providing External Quality Assessments of urine cytology analysis for over 10 years.
All the results collected have enabled us to model the dispersions expected at different concentration levels of white blood cells and red cells according to the three main methods: manual analysis, by Sysmex UF Analysers and by BECKMAN / IRIS IQ INSTRUMENTS.
We compared these dispersions observed in inter-laboratory tests.
Download a poster presented at the 2023 International Metrology Congress summarising this study.
To go further, find also the complete study to download: barèmes de fidélité en cytologie urinaire (in French)

Time-to-positivity in blood culture
Does the incubation period for detecting blood culture bottles as positive depend on the automated systems? Or on the bacterial species present in the blood?
The observations presented in this study are based on the results of 12 External Quality Assessments carried out between 2017 and 2020.
Download the Study of time-to-positivity in blood culture (in French)
